Madrid, May 12. (Europe Press) –
There can be up to 70 times more hydrogen in Earth’s core compared to the oceans It came in the form of water from the bombardment of materials from the solar system that happened billions of years ago.
This has been demonstrated through high pressure and high temperature experiments involving a diamond anvil and chemicals to simulate the core of the young Earth, which He showed for the first time that hydrogen can bind strongly to iron under extreme conditions.
Due to the extreme depths and the temperatures and pressures involved, we are not physically able to search very far directly from Earth. So, to dig deep into the Earth, researchers use techniques that include seismic data to determine things like the composition and density of materials found underground. The thing that has stood out since these types of measurements were made is that the core is mainly made of iron. But its density, especially that of the liquid fraction, is lower than expected.
This led researchers to believe that a large number of light elements must be present next to iron. For the first time, researchers have examined the behavior of water in laboratory experiments with metallic iron and silicate compounds that accurately mimic the interactions of metallic silicates (the primary mantle) during Earth’s formation. They found that when water meets iron, most of the hydrogen dissolves in the metal. Whereas, the oxygen reacts with the iron and turns into silicates.
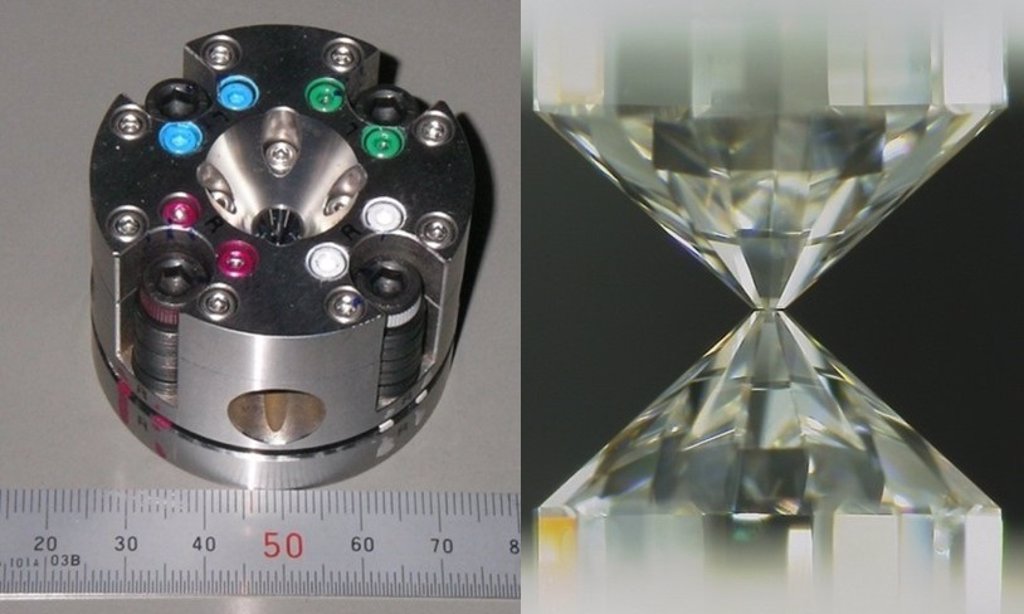
“In the temperatures and pressures we are used to on the surface, hydrogen does not bind to iron, but we wonder if that would be possible under more severe conditions,” said Shuh Tagawa, PhD student in the Department of Geosciences. Planetariums of the University of Tokyo while studying. “These extreme temperatures and pressures are not easy to reproduce, and the best way to achieve them in the laboratory is to use a diamond anvil. This can lead to pressures ranging from 30 to 60 GPa at temperatures from 3100 to 4600 K. This is a good simulation of core Earth formation. “
The team, led by Professor Kei Hirose, used mineral and hydrophobic silicates similar to those in the earth’s core and mantle respectively, and pressed them onto a diamond anvil while simultaneously heating the sample with To be. To find out what was happening in the sample, they used high-resolution images that incorporate a technique called secondary ion mass spectroscopy. This allowed them to confirm their hypothesis that hydrogen binds to iron, which explains the apparent deficiency of ocean water. Hydrogen is said to be iron-loving or iron-loving.
“This finding allows us to explore something that affects us in a very profound way,” said Hirose. It is a statement. “This hydrogen tells us that it is dorophilic at high pressure that much of the water that reached Earth in a massive bombardment during its formation could be in the core as hydrogen today. We estimate that there could be as many as 70 oceans of hydrogen locked in there. This remained on the surface like water, It is possible that the Earth would never have known the mainland, and that life as we know it would never have evolved“.

“Social media evangelist. Student. Reader. Troublemaker. Typical introvert.”


:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/7TXNTX4Z6ZADNGBBYTUT45QETM.jpg)
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/TR43PX4FQRCGJOYTK6DVVHHXGE.jpg)

More Stories
National Academy of Medicine and PAHO present reports of the Colombia General Physician Competency Forum – PAHO/WHO
Academic excellence in medical sciences is recognized at Granma.
Medical simulation has revolutionized the training of doctors in the country.